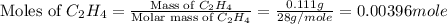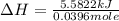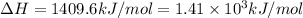Chemistry, 07.04.2020 15:12 kimberlylove387
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of ethylene (C 2H 4) was burned in this calorimeter, the temperature increased by 2.26 K. Calculate the energy of combustion for one mole of ethylene. a. -50.3 kJ/mol b. -1.41 x 103 kJ/mol c. -0.274 kJ/mol d. -624 kJ/mol e. -5.29 kJ/mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, cathydaves
What is the chemical formula of the following compound
Answers: 1

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 23.06.2019 02:30, babbity2009
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of et...
Questions in other subjects:

Mathematics, 22.01.2021 14:00


History, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00


Mathematics, 22.01.2021 14:00


Physics, 22.01.2021 14:00

Biology, 22.01.2021 14:00

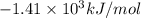
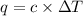
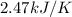
 = Change in temperature = 2.26 K
= Change in temperature = 2.26 K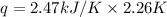
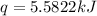
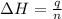
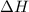 = energy of combustion for one mole of ethylene = ?
= energy of combustion for one mole of ethylene = ?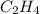 = 0.111 g
= 0.111 g