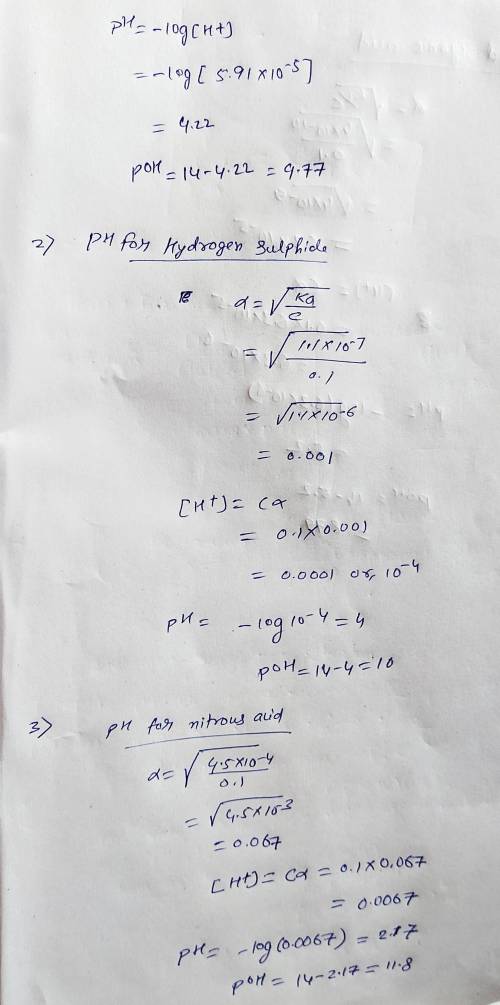
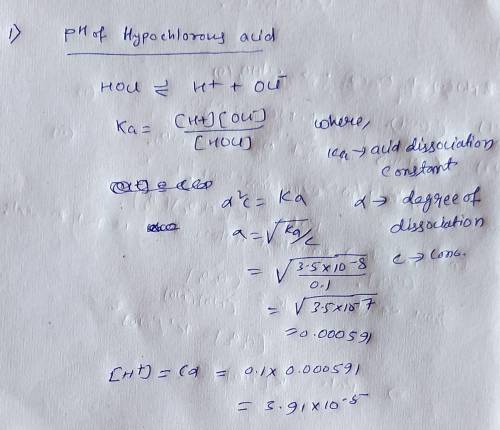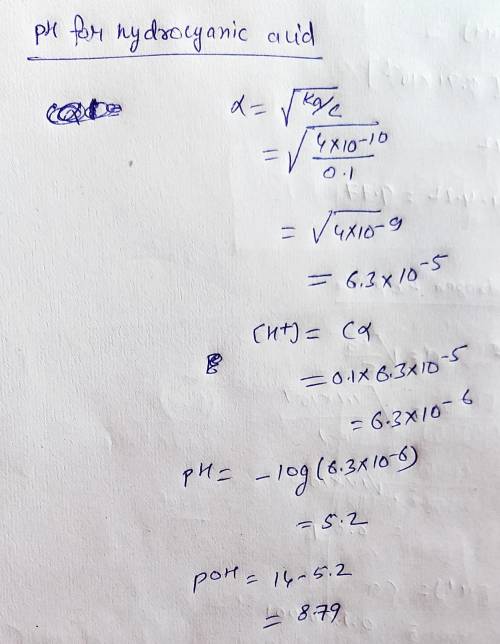The following is a list of weak acids and their Ka values. HOCl hypochlorous acid 3.5 x 10^-8H2S hydrogen sulfide 1.1 x 10^-7HCN hydrocyanic acid 4.0 x 10^-10HNO2 nitrous acid 4.5 x 10^-4a. Which acid given above is the strongest? Explain your choiceb. Write the Ka expression for the strongest acid. c. List the conjugate bases of each of the weak acids above and determine their Kb values. d. Which acid has the strongest conjugate base? Explain your choice. e. Determine the pH and pOH of a 0.1M solution of each.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 23.06.2019 00:00, alisonsolis155
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3

Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1
You know the right answer?
The following is a list of weak acids and their Ka values. HOCl hypochlorous acid 3.5 x 10^-8H2S hyd...
Questions in other subjects:

Mathematics, 21.11.2020 03:30

Mathematics, 21.11.2020 03:30



English, 21.11.2020 03:30

Mathematics, 21.11.2020 03:30

Mathematics, 21.11.2020 03:30



![Ka = \frac{[H^{+}][NO_{2} ^{-}] }{[HNO_{2}] }](/tpl/images/0585/7879/ebbcc.png)