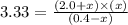Chemistry, 07.04.2020 02:47 lathwkuster
At certain temperature, kc for the reaction, PCl5 > PCl3 + Cl2, is equal to 3.33. After .20 mole of PCL5 and 1.0 mole of PCL3 are introduced iinto a 2.00 L evacuated chamber, calculate the equilibrium concentration of PCl5.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3


Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
At certain temperature, kc for the reaction, PCl5 > PCl3 + Cl2, is equal to 3.33. After .20 mole...
Questions in other subjects:

Mathematics, 23.09.2020 02:01

History, 23.09.2020 02:01


Chemistry, 23.09.2020 02:01

Chemistry, 23.09.2020 02:01

Mathematics, 23.09.2020 02:01

Biology, 23.09.2020 02:01


English, 23.09.2020 02:01

Mathematics, 23.09.2020 02:01

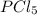 is, 0.16 M
is, 0.16 M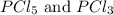
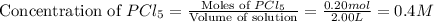
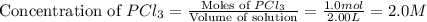
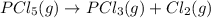
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0585/7341/73fe0.png)