
Wine goes bad soon after opening because the ethanol dissolved in it reacts with oxygen gas to form water and aqueous acetic acid , the main ingredient in vinegar. Calculate the moles of "oxygen" needed to produce "0.085" mol of "water". Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2


Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1
You know the right answer?
Wine goes bad soon after opening because the ethanol dissolved in it reacts with oxygen gas to form...
Questions in other subjects:

English, 23.06.2019 08:50

Mathematics, 23.06.2019 08:50





English, 23.06.2019 08:50

English, 23.06.2019 08:50

Mathematics, 23.06.2019 08:50

Chemistry, 23.06.2019 08:50

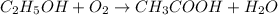
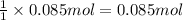 of oxygen gas
of oxygen gas 

