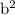Answer the following questions about the solubility of AgCl(s). The value of Ksp for AgCl(s) is 1.8 × 10−10.
Calculate the value of [Ag+] in a saturated solution of AgCl in distilled water.
The concentration of Cl−(aq) in seawater is 0.54 M.
Calculate the molar solubility of AgCl(s) in seawater.
Explain why AgCl(s) is less soluble in seawater than in distilled water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 04:30, ajsoccer1705
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 10:30, mv603177
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Answer the following questions about the solubility of AgCl(s). The value of Ksp for AgCl(s) is 1.8...
Questions in other subjects:

Mathematics, 27.06.2019 16:10

Mathematics, 27.06.2019 16:10



Mathematics, 27.06.2019 16:10


Mathematics, 27.06.2019 16:10

English, 27.06.2019 16:10

Mathematics, 27.06.2019 16:10

English, 27.06.2019 16:10

 M.
M.
 = x
= x  x
x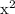

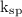 = b
= b