Chemistry, 06.04.2020 19:08 cassanovaanthony
Suppose 1.73g of barium nitrate is dissolved in 100.mL of a 63.0mM aqueous solution of sodium chromate.
Calculate the final molarity of nitrate anion in the solution. You can assume the volume of the solution doesn't change when the barium nitrate is dissolved in it. Be sure your answer has the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Suppose 1.73g of barium nitrate is dissolved in 100.mL of a 63.0mM aqueous solution of sodium chroma...
Questions in other subjects:

English, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

English, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

Mathematics, 14.09.2020 01:01

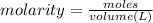
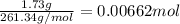
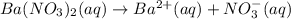
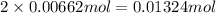 of nitrate ions
of nitrate ions