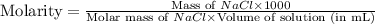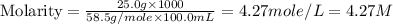Chemistry, 06.04.2020 18:19 Gghbhgy8716
25.0g of NaCl is placed in a 100.0ml volumetric flask. Enough water is added to dissolve the salt and then the volume is brought to 100.0ml total. What is the molar (moles/L or M) concentration of the NaCl?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2


Chemistry, 23.06.2019 10:30, lvoltin1073
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
25.0g of NaCl is placed in a 100.0ml volumetric flask. Enough water is added to dissolve the salt an...
Questions in other subjects:

History, 15.04.2020 01:05


Biology, 15.04.2020 01:05

English, 15.04.2020 01:05


Mathematics, 15.04.2020 01:05

Mathematics, 15.04.2020 01:05

Mathematics, 15.04.2020 01:05


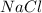 in the solution is, 4.27 M
in the solution is, 4.27 M