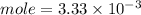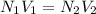Chemistry, 06.04.2020 18:02 hntnhtthnyt
Two burettes are set up. The first contains 0.15M NaOH and the second contains an HCl solution of unknown concentration. HCl is dispensed into the reaction flask and Phenolphthalein is added as an indicator. At the end of the titration, 22.2ml of NaOH and 9.45ml HCl were used.
Required:
A. Write a balanced equation for the Acid-Base reaction.
B. What is the total volume of NaOH used in the titration?
C. How many moles of NaOH was used?
D. What is the total volume of HCl used in the titration?
E. What is the final concentration of the HCl solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, HelenKellerwasaSlutt
The earth's moon is unusually large. two popular theories of the moon's origin include the "sister world" hypothesis, which states that the moon formed from the same materials as the earth, near enough to the earth that they fell into orbit around each other. a second theory is the "capture" hypothesis, in which the moon formed elsewhere in the solar system, and the earth's gravity pulled it into its orbit. studies of what the moon is made of indicate that some of its materials had to come from the earth or from the same area of the solar system where the earth had formed. at the same time, the moon does not contain much of the material that makes up the earth's core, so the moon could not have formed from the same materials as the earth. how do the two facts above affect the described theories of the moon's origin? a. they show that scientists will never agree on where the moon came from. b. they show that more experiments on moon formation need to be done. c. they show that no theory accounts for the existence of the moon. d. they show that neither theory is complete and entirely correct.
Answers: 2



Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
Two burettes are set up. The first contains 0.15M NaOH and the second contains an HCl solution of un...
Questions in other subjects:


English, 17.11.2020 22:20







Arts, 17.11.2020 22:20