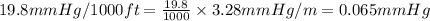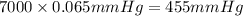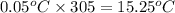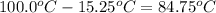The vapor pressure of a substance describes how readily molecules at the surface of the substance enter the gaseous phase. At the boiling point of a liquid, the liquid's vapor pressure is equal to or greater than the atmospheric pressure exerted on the surface of the liquid. Since the atmospheric pressure at higher elevations is lower than at sea level, the boiling point of water decreases as the elevation increases. The atmospheric pressure at sea level is 760 mmHg. This pressure decreases by 19.8 mmHg for every 1000-ft increase in elevation. Elevation Pressure0 m 760 mmHg1000 m 695 mmHg2000 m 630 mmHgThe boiling point of water decreases 0.05 ?C for every 1 mmHg drop in atmospheric pressure. A) What is the boiling point of water at an elevation of 7000m ?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1


Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 12:00, kenken2583
Jill is pushing a box across the floor. which represents the upward force perpendicular to the floor? a) fp b) ff c) fn d) fg
Answers: 1
You know the right answer?
The vapor pressure of a substance describes how readily molecules at the surface of the substance en...
Questions in other subjects:

History, 25.01.2022 01:10

Biology, 25.01.2022 01:10




Mathematics, 25.01.2022 01:10

Biology, 25.01.2022 01:10

Geography, 25.01.2022 01:10

Medicine, 25.01.2022 01:10

 meter
meter