
Chemistry, 06.04.2020 11:30 justice808
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at the same conditions of temperature and pressure.
2N2(g) + 3O2(g) 2N2O3(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 03:20, coollid876
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide...
Questions in other subjects:

Mathematics, 19.04.2020 04:26

Mathematics, 19.04.2020 04:26


Mathematics, 19.04.2020 04:26

History, 19.04.2020 04:26



Mathematics, 19.04.2020 04:26

Mathematics, 19.04.2020 04:26

History, 19.04.2020 04:26

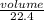
 = 0.67moles
= 0.67moles  = 1.01 moles of O₂
= 1.01 moles of O₂

