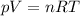What is the molar mass of a gas with a density of 3.50 g/L at a
temperature of 345 K and a pre...

Chemistry, 05.04.2020 22:22 raymondanthony3314
What is the molar mass of a gas with a density of 3.50 g/L at a
temperature of 345 K and a pressure of 0.950 atm? (Write your
answer whole number e. g. 345)
g/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 10:30, tjjjjjjjjjjjjjjjjjjj
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Questions in other subjects:





Mathematics, 25.11.2021 09:50

Physics, 25.11.2021 09:50

Chemistry, 25.11.2021 09:50

Mathematics, 25.11.2021 09:50


History, 25.11.2021 09:50