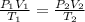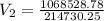During laparoscopic surgery, carbon dioxide gas is used to expand the abdomen to help create a larger working space.
If 4.40 L of CO2 gas at 19 ∘C at 783 mmHg is used, what is the final volume, in liters, of the gas at 37 ∘C and a pressure of 735 mmHg, if the amount of CO2 does not change?
Express your answer with the appropriate units.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3


You know the right answer?
During laparoscopic surgery, carbon dioxide gas is used to expand the abdomen to help create a large...
Questions in other subjects:


History, 04.08.2019 10:00


Mathematics, 04.08.2019 10:00

Mathematics, 04.08.2019 10:00

Physics, 04.08.2019 10:00


Mathematics, 04.08.2019 10:00