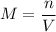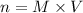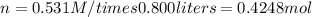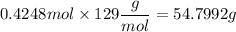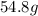Chemistry, 05.04.2020 17:39 laceysmith2i023
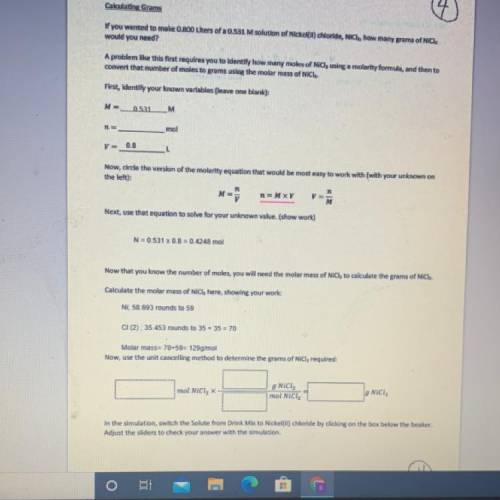
If you wanted to make 0.800 Liters of a 0.531 M solution of Nickel(II) chloride, NiCl2, how many grams of NiCIZ
would you need? Use the unit canceling method to determine the grams of NiCl2
Molar mass= 129g/mol. Moles = 0.4248
.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
If you wanted to make 0.800 Liters of a 0.531 M solution of Nickel(II) chloride, NiCl2, how many gra...
Questions in other subjects:

Mathematics, 16.07.2019 20:00

Spanish, 16.07.2019 20:00


Chemistry, 16.07.2019 20:00



Physics, 16.07.2019 20:00


Mathematics, 16.07.2019 20:00

Biology, 16.07.2019 20:00