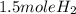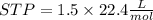Chemistry, 05.04.2020 05:17 screamqueen
2Li + H2SO4=Li2SO4 + H2 How many liters of hydrogen gas, H2 at STP can be produced from 3.0 moles of Li? The molar volume of a gas at STP is 22.4L/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2


Chemistry, 23.06.2019 04:10, NavyCo
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
2Li + H2SO4=Li2SO4 + H2 How many liters of hydrogen gas, H2 at STP can be produced from 3.0 moles of...
Questions in other subjects:

Mathematics, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00

History, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00


Mathematics, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00

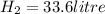
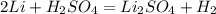
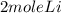 ⇔
⇔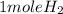
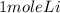 ⇔
⇔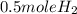
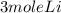 ⇔
⇔