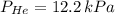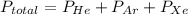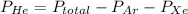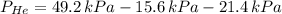Chemistry, 04.04.2020 21:30 maisonsuperman5321
A mixture of helium, argon, and xenon gases are present in a container. What is the partial pressure of helium if the total pressure is 49.2 kPa and the partial pressure of Ar is 15.6 kPa and 21,4 kPa for Xe?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 23.06.2019 05:40, 19youngr
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2

You know the right answer?
A mixture of helium, argon, and xenon gases are present in a container. What is the partial pressure...
Questions in other subjects:

Mathematics, 31.12.2019 09:31



English, 31.12.2019 09:31


Social Studies, 31.12.2019 09:31

Mathematics, 31.12.2019 09:31

Mathematics, 31.12.2019 09:31

Mathematics, 31.12.2019 09:31