Chemistry, 04.04.2020 21:02 kevinhill185
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is produced by water displacement. If the lab temperature is 24 C and the atmospheric pressure is 742.1 mm Hg, how many grams of hydrogen are produced? Water vapor pressure is 22.4 mm Hg at 24 C.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:00, ItzAquaZ1449
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2

Chemistry, 23.06.2019 08:40, mathisaqeosmw
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3

Chemistry, 23.06.2019 09:20, dncs9157
Four statements about the development of the atomic model are shown below. a: electrons have wavelike properties. b: atoms have small, negatively charged particles. c. the center of an atom is a small, dense nucleus. d: atoms are hard, indivisible spheres. which order of statements represents the historical development of the atomic model? c-d-a-b c-d-b-a d— в-а — с d-b-c-a
Answers: 1

Chemistry, 23.06.2019 10:30, piratesfc02
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is...
Questions in other subjects:

English, 28.01.2020 01:31






Health, 28.01.2020 01:31



Mathematics, 28.01.2020 01:31

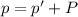
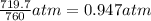
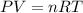 ( Ideal gas equation)
( Ideal gas equation)