
Chemistry, 04.04.2020 19:43 valtrump3256
A 500.0 g sample of Al2(SO4)3 is reacted with 450.0 g of Ca(OH)2. A total of 596 g of CaSO4 is produced. What is the limiting reagent in this reaction, and how many moles of excess reagent are unreacted? Al2(SO4)3(aq) + 3Ca(OH)2(aq) -> 2Al(OH)3(s) + 3CaSO4(s)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
A 500.0 g sample of Al2(SO4)3 is reacted with 450.0 g of Ca(OH)2. A total of 596 g of CaSO4 is produ...
Questions in other subjects:

Physics, 18.12.2020 17:30


Mathematics, 18.12.2020 17:30






Mathematics, 18.12.2020 17:30

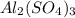 and number of moles of excess reagent is, 1.69 moles
and number of moles of excess reagent is, 1.69 moles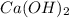 = 450.0 g
= 450.0 g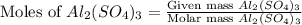
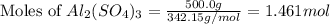
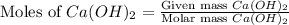
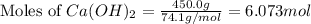
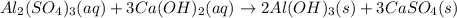
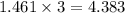 moles of
moles of 

