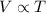Chemistry, 04.04.2020 18:25 ravenjade2395
Jorge inflates a beach ball to a volume of 4L in his air-conditioned house where the temperature is 18℃. That afternoon he takes the beach ball to the beach with some friends. The temperature at the beach is 32℃, and the air pressure at the beach is the same as it was at Jorge's house. What will happen to Jorge's beach ball when he is at the beach? Choose the claim that best answers the question: * 1 point The beach ball will get smaller at the beach because the molecules are moving slower. The beach ball will stay the same size at the beach because the pressure is constant. The beach ball will get larger at the beach because the molecules are moving faster. The beach ball will get larger at the beach because the pressure will cause it to expand. The beach ball will get smaller at the beach because the molecules will collide less often.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1


Chemistry, 22.06.2019 23:50, datboyjulio21
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
Jorge inflates a beach ball to a volume of 4L in his air-conditioned house where the temperature is...
Questions in other subjects:


Mathematics, 19.02.2021 23:10

Mathematics, 19.02.2021 23:10



Mathematics, 19.02.2021 23:10


SAT, 19.02.2021 23:10

English, 19.02.2021 23:10

Mathematics, 19.02.2021 23:10