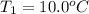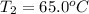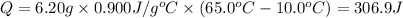Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
Calculate the energy absorbed when 6.20 g of aluminum is heated from 10.0°C to 65.0°C if the specifi...
Questions in other subjects:

Arts, 20.04.2021 17:40



English, 20.04.2021 17:40


Health, 20.04.2021 17:40




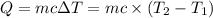
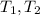 : Initial and final temperature of the substance
: Initial and final temperature of the substance