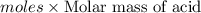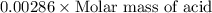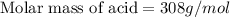Chemistry, 04.04.2020 13:02 lnbrown5633
A 0.881 g sample of a diprotic acid is dissolved in water and titrated with 0.160 M NaOH . What is the molar mass of the acid if 35.8 mL of the NaOH solution is required to neutralize the sample

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2


Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2
You know the right answer?
A 0.881 g sample of a diprotic acid is dissolved in water and titrated with 0.160 M NaOH . What is t...
Questions in other subjects:


Chemistry, 26.06.2019 20:30


Biology, 26.06.2019 20:30



Mathematics, 26.06.2019 20:30



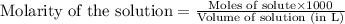 .....(1)
.....(1)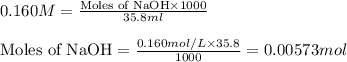
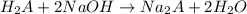
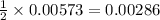 moles of diprotic acid
moles of diprotic acid