
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: (g)(g)(g) In the second step, ammonia and oxygen react to form nitric acid and water: (g)(g)(g)(g) Write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. Be sure your equation is balanced.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prep...
Questions in other subjects:







Health, 01.02.2021 22:40

Mathematics, 01.02.2021 22:40


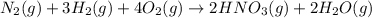
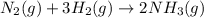 (1)
(1)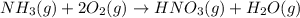
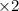
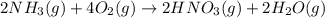 (2)
(2)

