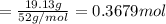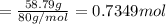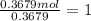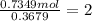Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, 20alondra04
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1


You know the right answer?
A 19.13 gram sample of chromium is heated in the presence of excess bromine. A metal bromide is form...
Questions in other subjects:

Mathematics, 17.12.2020 14:00

History, 17.12.2020 14:00

Physics, 17.12.2020 14:00






Biology, 17.12.2020 14:00

History, 17.12.2020 14:00

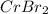 .
.