
Chemistry, 04.04.2020 12:08 ErrorNameTaken505
Lead (II) sulfide, PbS, reacts with oxygen gas to produce lead (II) oxide and sulfur dioxide. If 0.750 moles of O2 were used during this chemical reaction, how many grams of lead (II) oxide would be produced?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1



Chemistry, 24.06.2019 03:00, alyssashae1818
How many atoms in 0.50 moles ag? have to show work
Answers: 1
You know the right answer?
Lead (II) sulfide, PbS, reacts with oxygen gas to produce lead (II) oxide and sulfur dioxide. If 0.7...
Questions in other subjects:



Mathematics, 28.10.2020 20:10




Biology, 28.10.2020 20:10



Health, 28.10.2020 20:10

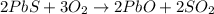
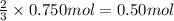 of lead (II) oxide
of lead (II) oxide

