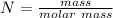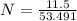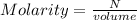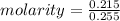Chemistry, 04.04.2020 11:00 lilpump3506
A solution is created by dissolving 11.5 grams of ammonium chloride in enough water to make 255 mL of solution. How many moles of ammonium chloride are present in the resulting solution

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
A solution is created by dissolving 11.5 grams of ammonium chloride in enough water to make 255 mL o...
Questions in other subjects:


Mathematics, 01.07.2019 04:30


Mathematics, 01.07.2019 04:30





Biology, 01.07.2019 04:30