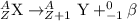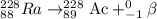Because of the radioactive decay of uranium and thorium in rocks and soil, radium-228, a decay product of Thorium-232, can be found in drinking water. This isotope has a half-life of 5.75 years and an atomic number of 88. If Ra-228 undergoes beta decay, what would the atomic number of the new element be? What would the mass number of this isotope be? Explain your reasoning (e. g. Explain what happens during beta decay).

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1
You know the right answer?
Because of the radioactive decay of uranium and thorium in rocks and soil, radium-228, a decay produ...
Questions in other subjects:

English, 10.09.2020 18:01

Mathematics, 10.09.2020 18:01

Mathematics, 10.09.2020 18:01

Mathematics, 10.09.2020 18:01

Mathematics, 10.09.2020 18:01

Mathematics, 10.09.2020 18:01

Biology, 10.09.2020 18:01

Mathematics, 10.09.2020 18:01

Mathematics, 10.09.2020 18:01

Mathematics, 10.09.2020 18:01