
Chemistry, 04.04.2020 11:05 ellaemtagedeane
When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equation for this reaction is:
Fe2O3 (s) + 2Al (s) > Al2O3 (s) + 2Fe (s)
If 6 moles of aluminum react:
i. The reaction consumes moles of iron(III) oxide.
ii. The reaction produces moles of aluminum oxide and moles of iron.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1


You know the right answer?
When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equati...
Questions in other subjects:





History, 30.06.2019 01:30

Mathematics, 30.06.2019 01:30

Mathematics, 30.06.2019 01:30

Biology, 30.06.2019 01:30


Chemistry, 30.06.2019 01:30

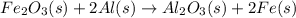
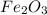
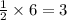 moles of
moles of 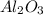
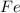
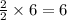 moles of
moles of 

