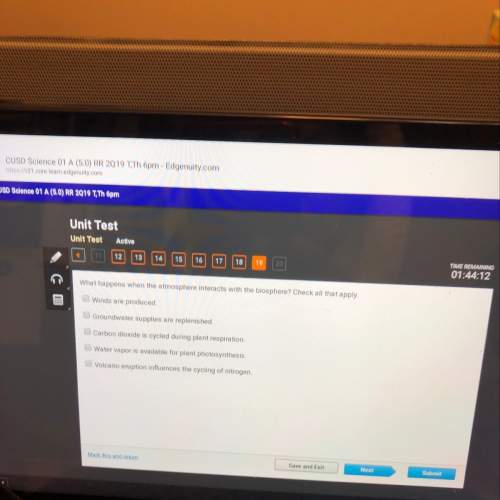
Consider the following elementary reaction: (CH₃)₃CBr (aq) → (CH₃)₃⁺ (aq)+ Br⁻ (aq) Suppose we let k₁ stand for the rate constant of this reaction and k₋₁stand for the rate constant of the reverse reaction. Write an expression that gives the equilibrium concentration of (CH₃)₃C⁺ in terms of k₁, k₋₁, and the equilibrium concentrations of (CH₃)₃CBr and Br⁻.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2
You know the right answer?
Consider the following elementary reaction: (CH₃)₃CBr (aq) → (CH₃)₃⁺ (aq)+ Br⁻ (aq) Suppose we let k...
Questions in other subjects:

Mathematics, 16.04.2021 21:40


History, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40

Mathematics, 16.04.2021 21:40


Mathematics, 16.04.2021 21:40