
Chemistry, 01.02.2020 09:45 TH3L0N3W0LF
Calculate the freezing point of a 2.6-molal aqueous sucrose solution. the freezing point depression constant for water is 1.86 degrees c/molal.
a.) 4.8 °c
b.) 1.4 °c
c.) -1.4 °c
d.) -4.8 °c

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Calculate the freezing point of a 2.6-molal aqueous sucrose solution. the freezing point depression...
Questions in other subjects:

Mathematics, 18.09.2019 19:30


History, 18.09.2019 19:30

Mathematics, 18.09.2019 19:30



Mathematics, 18.09.2019 19:30


Mathematics, 18.09.2019 19:30

Business, 18.09.2019 19:30

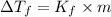
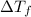 = change in freezing point
= change in freezing point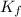 = freezing point constant =
= freezing point constant = 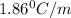
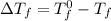
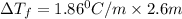
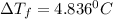
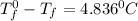
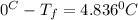
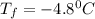
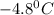 .
.

