

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 17:40, monkey2865
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

You know the right answer?
A chemist dissolves 751.mg of pure nitric acid in enough water to make up 290.mL of solution. Calcul...
Questions in other subjects:

English, 20.07.2019 02:30

Mathematics, 20.07.2019 02:30




English, 20.07.2019 02:30

History, 20.07.2019 02:30

Social Studies, 20.07.2019 02:30


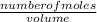

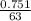 = 0.012mole
= 0.012mole  = 0.041moldm⁻³
= 0.041moldm⁻³


