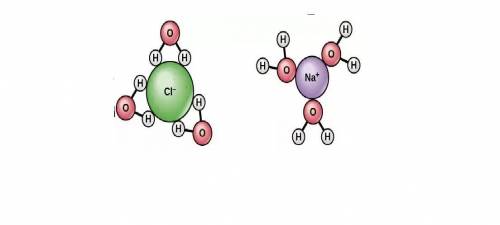
Which of these best explains why the hydrogen atoms in a water molecule are attracted to cl− ions in sodium chloride (nacl)?
a. the hydrogen atoms have +1 charges.
b. the hydrogen atoms share electrons equally with the oxygen atom.
c. the hydrogen atoms have partial positive charges.
d. the hydrogen atoms form covalent bonds with the chloride ions.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, umimgoingtofail
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 23.06.2019 01:20, cedricevans41p4j3kx
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Which of these best explains why the hydrogen atoms in a water molecule are attracted to cl− ions in...
Questions in other subjects:

History, 24.02.2021 18:30








Mathematics, 24.02.2021 18:30

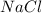 by getting between the oppositely charged ions of solute and thus decreasing the forces of attraction between the ions. The positively charged
by getting between the oppositely charged ions of solute and thus decreasing the forces of attraction between the ions. The positively charged 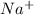 ions are attracted to positive pole of water which is hydrogen and the negatively charged
ions are attracted to positive pole of water which is hydrogen and the negatively charged 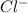 is attracted to negative pole of water which is oxygen.
is attracted to negative pole of water which is oxygen.