
Chemistry, 04.04.2020 02:33 nate102201
Potassium hydroxide is very soluble in water, resulting in extremely basic solutions. A 121g sample KOH is dissolved in water at 25∘C to make up 100.0mL of solution. The molar mass of KOH is 56.11gmol. What is the pH of the solution at 25.0∘C?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 23.06.2019 07:00, jaydenboi604
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1

Chemistry, 23.06.2019 10:20, Thejollyhellhound20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3
You know the right answer?
Potassium hydroxide is very soluble in water, resulting in extremely basic solutions. A 121g sample...
Questions in other subjects:

English, 10.12.2020 14:00

Business, 10.12.2020 14:00

Spanish, 10.12.2020 14:00

Engineering, 10.12.2020 14:00


Chemistry, 10.12.2020 14:00

Mathematics, 10.12.2020 14:00


Mathematics, 10.12.2020 14:00

History, 10.12.2020 14:00

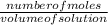
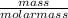 =
= 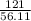 = 2.16mole
= 2.16mole 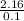 = 21.6moldm⁻³
= 21.6moldm⁻³

