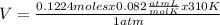Chemistry, 04.04.2020 01:45 michaelgold1
The equation for the metabolic breakdown of glucose (C6H12O6) is the same as the equation for the combustion of glucose in air: C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) Calculate the volume of CO2 produced at 37°C and 1.00 atm when 3.68 g of glucose is used up in the reaction.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
The equation for the metabolic breakdown of glucose (C6H12O6) is the same as the equation for the co...
Questions in other subjects:

Social Studies, 12.01.2020 05:31





Mathematics, 12.01.2020 05:31




Mathematics, 12.01.2020 05:31

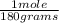 = 0.0204 moles
= 0.0204 moles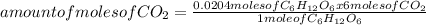
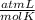 T= 37 C= 310 K (being 0 C= 273 K)
T= 37 C= 310 K (being 0 C= 273 K)