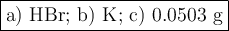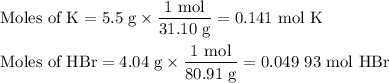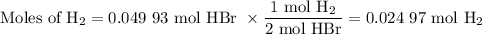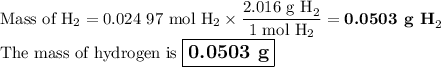2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hyd...

2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hydrogen gas(H₂)
●a). What is the limiting reactant?
●b.)What is the excess reactant?
2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hydrogen gas(H₂)
●a). What is the limiting reactant?
●b.)What is the excess reactant?
2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hydrogen gas(H₂)
●a). What is the limiting reactant?
●b.)What is the excess reactant?
●C.)How much product is produced?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, XxrazorxX11
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 23.06.2019 00:00, scottykinkade7860
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1
You know the right answer?
Questions in other subjects:



Mathematics, 03.02.2020 08:02

Mathematics, 03.02.2020 08:02


Mathematics, 03.02.2020 08:02

Mathematics, 03.02.2020 08:02

Mathematics, 03.02.2020 08:02