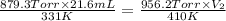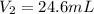Chemistry, 03.04.2020 04:31 wolfiewolffromsketch
A 21.6 ml ball has a pressure of 879.3 torr at 331 K. If the temperatire spikes 410 K and the pressure increases to 956.2 torr what is the volume of the bal

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
A 21.6 ml ball has a pressure of 879.3 torr at 331 K. If the temperatire spikes 410 K and the pressu...
Questions in other subjects:

Social Studies, 18.10.2021 19:50




Computers and Technology, 18.10.2021 19:50


Mathematics, 18.10.2021 19:50



History, 18.10.2021 19:50


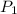 = initial pressure inside a ball = 879.3 Torr
= initial pressure inside a ball = 879.3 Torr = final pressure inside a ball = 956.2 Torr
= final pressure inside a ball = 956.2 Torr = initial volume of ball =
= initial volume of ball = 
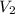 = final volume of gas = ?
= final volume of gas = ? = initial temperature of gas =
= initial temperature of gas = 
 = final temperature of gas =
= final temperature of gas =