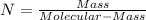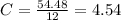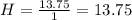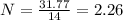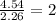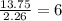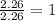Chemistry, 03.04.2020 03:29 danding1593
What is the empirical formula for a compound that is 54.48% carbon, 13.75% hydrogen, and 31.77 % nitrogen?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 02:30, babbity2009
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 02:40, towelmearowel
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 04:00, winterblanco
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
You know the right answer?
What is the empirical formula for a compound that is 54.48% carbon, 13.75% hydrogen, and 31.77 % nit...
Questions in other subjects:

Mathematics, 16.12.2020 03:30

Mathematics, 16.12.2020 03:30

Mathematics, 16.12.2020 03:30

Spanish, 16.12.2020 03:30


Health, 16.12.2020 03:30

Mathematics, 16.12.2020 03:30

Social Studies, 16.12.2020 03:30


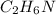 .
.