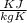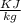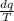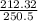Chemistry, 02.04.2020 04:26 tifftiff22
Using the relation dS = (δQ/T)int rev for the definition of entropy, calculate the change in the specific entropy of R-134a as it is heated at a constant pressure of 120 kPa from a saturated liquid to a saturated vapor. Use the tables for R-134a.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3


Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
Using the relation dS = (δQ/T)int rev for the definition of entropy, calculate the change in the spe...
Questions in other subjects:




Health, 27.04.2021 18:20

Social Studies, 27.04.2021 18:20

English, 27.04.2021 18:20




Mathematics, 27.04.2021 18:20