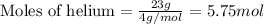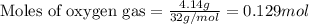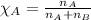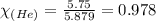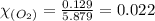Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:50, nikoidurrant
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
You know the right answer?
A 15.0-L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 23.0 g of He and 4.1...
Questions in other subjects:




Mathematics, 22.02.2021 02:40


Biology, 22.02.2021 02:40

English, 22.02.2021 02:40

Mathematics, 22.02.2021 02:40


English, 22.02.2021 02:40

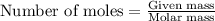 .....(1)
.....(1)