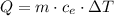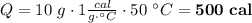(using the formula given in the lesson, solve the following problem.)
the specific heat of wat...

Chemistry, 29.08.2019 12:50 georgeonnatreev2275
(using the formula given in the lesson, solve the following problem.)
the specific heat of water is 1.00 cal/g °c. if 10 g of water were heated and the water changed by 50°c, how many calories of heat energy would be absorbed by the water?
calories
500
50
10

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 00:30, hdhshshs741
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 04:10, NavyCo
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 09.06.2020 12:57


English, 09.06.2020 12:57



Mathematics, 09.06.2020 12:57