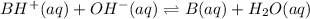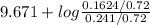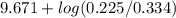Chemistry, 02.04.2020 02:30 dontcareanyonemo
A buffer that contains 0.17 M of a base, B and 0.39 M of its conjugate acid BH+, has a pH of 9.31. What is the pH after 0.02 mol of Ba(OH)2 are added to 0.72 L of the solution?

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
A buffer that contains 0.17 M of a base, B and 0.39 M of its conjugate acid BH+, has a pH of 9.31. W...
Questions in other subjects:

Geography, 17.10.2021 03:50

Mathematics, 17.10.2021 03:50

Social Studies, 17.10.2021 03:50


Mathematics, 17.10.2021 03:50

English, 17.10.2021 03:50




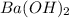 are added to 0.72 L of the solution is 9.5.
are added to 0.72 L of the solution is 9.5.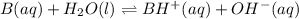
![pK_{a} + log \frac{[B]}{[BH^{+}]}](/tpl/images/0577/1202/d0a56.png)
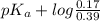
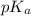 = 9.31 + 0.361
= 9.31 + 0.361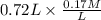
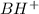 is as follows.
is as follows.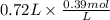
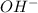 as follows.
as follows.