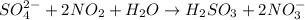Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
SO42- 2NO2 H2OH2SO3 2NO3- In the above redox reaction, using oxidation numbers to identify the eleme...
Questions in other subjects:


History, 21.09.2019 19:30


Chemistry, 21.09.2019 19:30

Mathematics, 21.09.2019 19:30

English, 21.09.2019 19:30

Mathematics, 21.09.2019 19:30



Mathematics, 21.09.2019 19:30

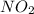 act as reducing agent and
act as reducing agent and 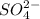 act as an oxidizing agent.
act as an oxidizing agent.