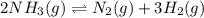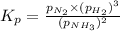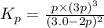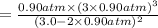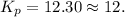Chemistry, 02.04.2020 01:21 leannaadrian
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 500. ML flask with 3.0 atm of ammonia gas, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 0.90 atm . Calculate the pressure equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to 2 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions in other subjects:





Social Studies, 24.09.2019 22:30

Spanish, 24.09.2019 22:30


Biology, 24.09.2019 22:30