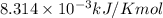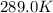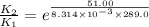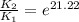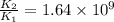Chemistry, 01.04.2020 22:23 milkshakegrande101
The presence of a catalyst provides a reaction pathway in which the activation energy of a reaction is reduced by 51.00 kJ ⋅ mol − 1 51.00 kJ⋅mol−1 . Uncatalyzed: A ⟶ B A⟶B E a = 136.00 kJ ⋅ mol − 1 Ea=136.00 kJ⋅mol−1 Catalyzed: A ⟶ B A⟶B E a = 85.00 k J ⋅ mol − 1 Ea=85.00 kJ⋅mol−1

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, monnicawilliam
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2


You know the right answer?
The presence of a catalyst provides a reaction pathway in which the activation energy of a reaction...
Questions in other subjects:

Mathematics, 20.01.2020 02:31

Mathematics, 20.01.2020 02:31

Mathematics, 20.01.2020 02:31


Spanish, 20.01.2020 02:31


Biology, 20.01.2020 02:31

Social Studies, 20.01.2020 02:31


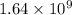
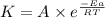
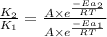
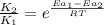
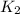 = rate of reaction with catalyst
= rate of reaction with catalyst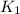 = rate of reaction without catalyst
= rate of reaction without catalyst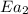 = activation energy with catalyst
= activation energy with catalyst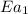 = activation energy without catalyst
= activation energy without catalyst