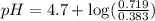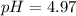Chemistry, 01.04.2020 22:21 jaallen3679
Calculate the pH of a solution made by adding 59 g of sodium acetate, NaCH3COO, to 23 g of acetic acid, CH3COOH, and dissolving in water to make 400. mL of solution. Hint given in feedback.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


You know the right answer?
Calculate the pH of a solution made by adding 59 g of sodium acetate, NaCH3COO, to 23 g of acetic ac...
Questions in other subjects:

Mathematics, 09.01.2020 22:31




History, 09.01.2020 22:31


Health, 09.01.2020 22:31


Mathematics, 09.01.2020 22:31

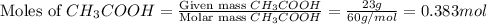
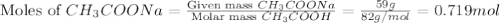
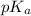 .
.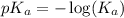
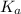 in this expression, we get:
in this expression, we get: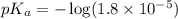
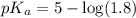
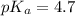
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0576/5065/e961a.png)
![pH=pK_a+\log \frac{[CH_3COONa]}{[CH_3COOH]}](/tpl/images/0576/5065/023d4.png)