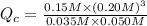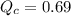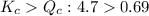Chemistry, 01.04.2020 19:33 doralisaponte79851
The first step in the industrial synthesis of hydrogen is the reaction of steam and methane to give synthesis gas, a mixture of carbon monoxide and hydrogen. h2o(g)+ch4(g)⇌co(g)+3h2(g) kc = 4.7 at 1400k. a mixture of reactants and products at 1400k contains 0.035 m h2o, 0.050m ch4, 0.15 m co, and 0.20 m h2.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
The first step in the industrial synthesis of hydrogen is the reaction of steam and methane to give...
Questions in other subjects:

Mathematics, 15.12.2021 01:40




Mathematics, 15.12.2021 01:40


Mathematics, 15.12.2021 01:40

Mathematics, 15.12.2021 01:40

History, 15.12.2021 01:40

Mathematics, 15.12.2021 01:40

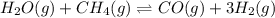
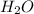 , 0.050 M
, 0.050 M 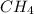 , 0.15 M
, 0.15 M 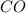 , and 0.20 M
, and 0.20 M 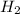 .
.
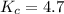
![[H_2O]=0.035 M](/tpl/images/0575/9166/507ff.png)
![[CH_4]=0.050 M](/tpl/images/0575/9166/de553.png)
![[CO]=0.15 M](/tpl/images/0575/9166/629de.png)
![[H_2]=0.20 M](/tpl/images/0575/9166/1d49e.png)
![Q_c=\frac{[CO][H_2]^3}{[H_2O][CH_4]}](/tpl/images/0575/9166/7c8ad.png)