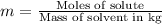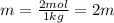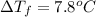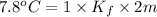Chemistry, 01.04.2020 18:36 kwarwick0915
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freezes at 7.8°c below its normal freezing point. what is the molal freezing-point constant of the unknown solvent? suggest a possible identity of the solvent.

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 04:00, zakarycrane8101
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freez...
Questions in other subjects:

Mathematics, 21.10.2020 22:01

Social Studies, 21.10.2020 22:01

Social Studies, 21.10.2020 22:01

History, 21.10.2020 22:01




Mathematics, 21.10.2020 22:01

Chemistry, 21.10.2020 22:01

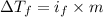
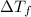 =depression in freezing point =
=depression in freezing point = 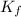 = freezing point constant
= freezing point constant