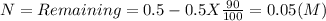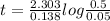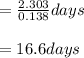Chemistry, 01.04.2020 16:11 kenyasutton10
Iodine-131 is a radioactive isotope that is used to diagnose and treat some forms of thyroid cancer. Iodine-131 decays to xenon-131 according to the equation: I-131⟶Xe-131+electron The decay is first-order with a rate constant of 0.138 d−1. How many days will it take for 90% of the iodine−131 in a 0.500 M solution of this substance to decay to Xe-131?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, sillslola816oxb5h7
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1
You know the right answer?
Iodine-131 is a radioactive isotope that is used to diagnose and treat some forms of thyroid cancer....
Questions in other subjects:

History, 04.12.2020 18:40

Mathematics, 04.12.2020 18:40

Advanced Placement (AP), 04.12.2020 18:40

World Languages, 04.12.2020 18:40

Chemistry, 04.12.2020 18:40

Mathematics, 04.12.2020 18:40

Mathematics, 04.12.2020 18:40

Social Studies, 04.12.2020 18:40

Mathematics, 04.12.2020 18:40

Mathematics, 04.12.2020 18:40

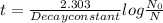 ----------------------------------(1)
----------------------------------(1)