
Chemistry, 01.04.2020 01:55 olivasm5626
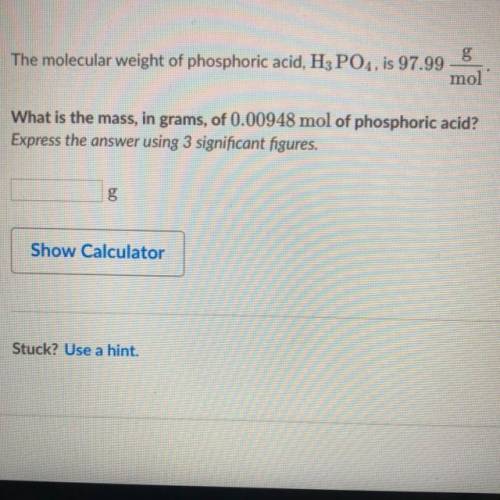
The molecular weight of phosphoric acid, H 3 PO 4 , is 97.99 * g/(mol) What is the mass, in grams, of 0.00948 mol of phosphoric acid?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 23.06.2019 01:00, only1cache
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
The molecular weight of phosphoric acid, H 3 PO 4 , is 97.99 * g/(mol) What is the mass, in grams, o...
Questions in other subjects:


Mathematics, 23.06.2019 02:00




History, 23.06.2019 02:00

Mathematics, 23.06.2019 02:00



Mathematics, 23.06.2019 02:00



