
Chemistry, 31.03.2020 21:25 mckenziew6969
4 NH3 + 5 O2 > 4 NO + 6 H2O
How many moles and how many grams of oxygen (O2) are needed to react with 56.8 grams
of ammonia by this reaction?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tiniecisneros28
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1


Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 23.06.2019 16:30, sandygarcia65
Which of the following is a way carbon enters the atmosphere volcanic activity photosynthesis deposition of settlement burial of biomass hep hurrryy
Answers: 1
You know the right answer?
4 NH3 + 5 O2 > 4 NO + 6 H2O
How many moles and how many grams of oxygen (O2) are needed to...
How many moles and how many grams of oxygen (O2) are needed to...
Questions in other subjects:


Mathematics, 13.09.2019 04:30








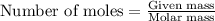 .....(1)
.....(1)
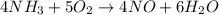
 of oxygen gas
of oxygen gas


