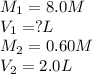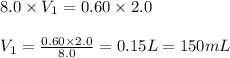Chemistry, 31.03.2020 19:17 BakedBiscuit6896
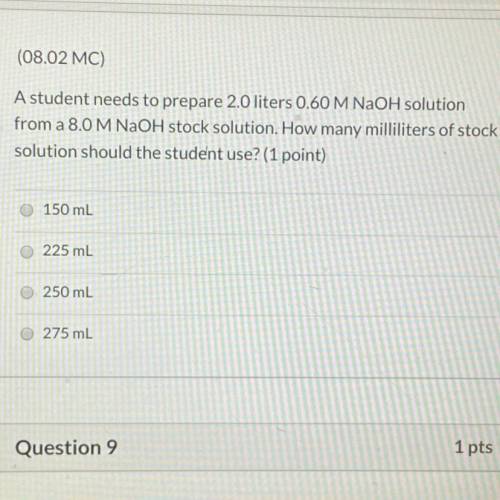
A student needs to prepare 2.0 liters 0.60 M NaOH solution from a 8.0 M NaOH stock solution. How many milliliters of stock solution should the student use?(1 point)
A.150 mL
B.225 ml
C.250 mL
D.275 mL

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:20, zymikaa00
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
A student needs to prepare 2.0 liters 0.60 M NaOH solution from a 8.0 M NaOH stock solution. How man...
Questions in other subjects:

Mathematics, 10.07.2019 14:50

Mathematics, 10.07.2019 14:50

History, 10.07.2019 14:50

History, 10.07.2019 14:50

Mathematics, 10.07.2019 14:50


Mathematics, 10.07.2019 14:50

Social Studies, 10.07.2019 14:50

Mathematics, 10.07.2019 14:50

Mathematics, 10.07.2019 14:50

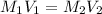
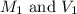 are the molarity and volume of the stock NaOH solution
are the molarity and volume of the stock NaOH solution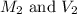 are the molarity and volume of diluted NaOH solution
are the molarity and volume of diluted NaOH solution