HELP PLEASE
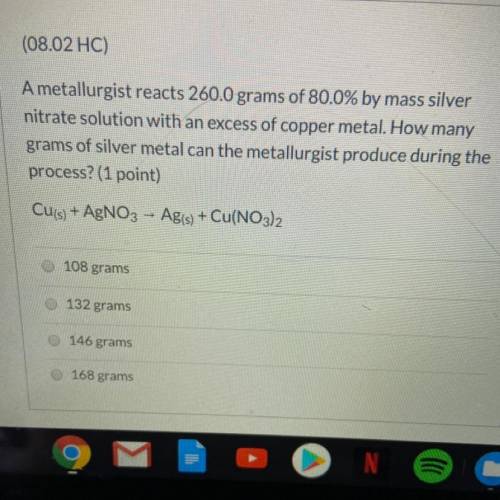
A metallurgist reacts 260.0 grams of 80.0% by mass silver nitrate solution w...

Chemistry, 31.03.2020 18:56 sparkyjones02
HELP PLEASE
A metallurgist reacts 260.0 grams of 80.0% by mass silver nitrate solution with an excess of copper metal. How many grams of silver metal can the metallurgist produce during the process?
Cu(s) + AgNO3 - Ag(s) + Cu(NO3)2
Answers
A.108 grams
B.132 grams
C.146 grams
D.168 grams

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, vlactawhalm29
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 23.06.2019 02:40, sherlock19
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 29.07.2021 21:40

Mathematics, 29.07.2021 21:40

Mathematics, 29.07.2021 21:40

Mathematics, 29.07.2021 21:40


Mathematics, 29.07.2021 21:40


English, 29.07.2021 21:40

Mathematics, 29.07.2021 21:40



